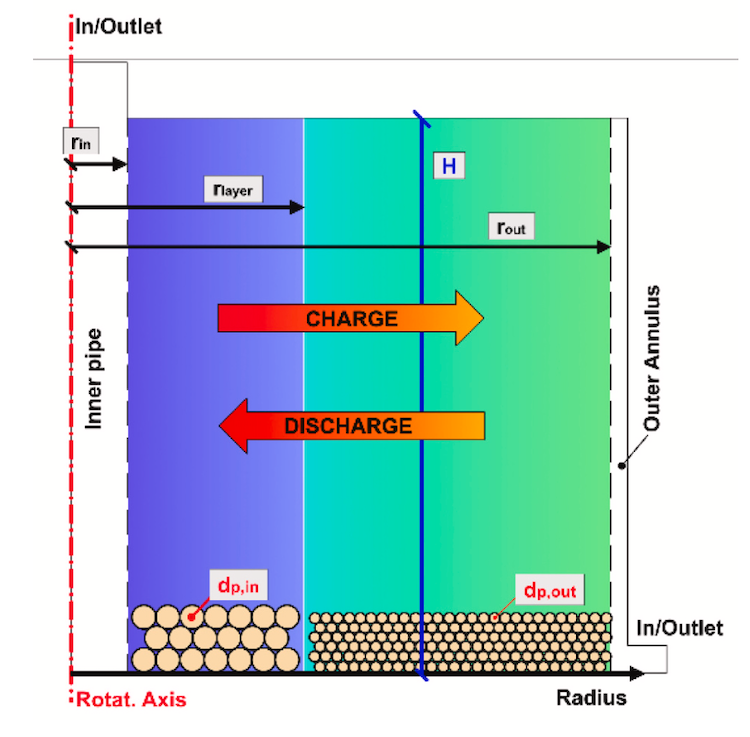
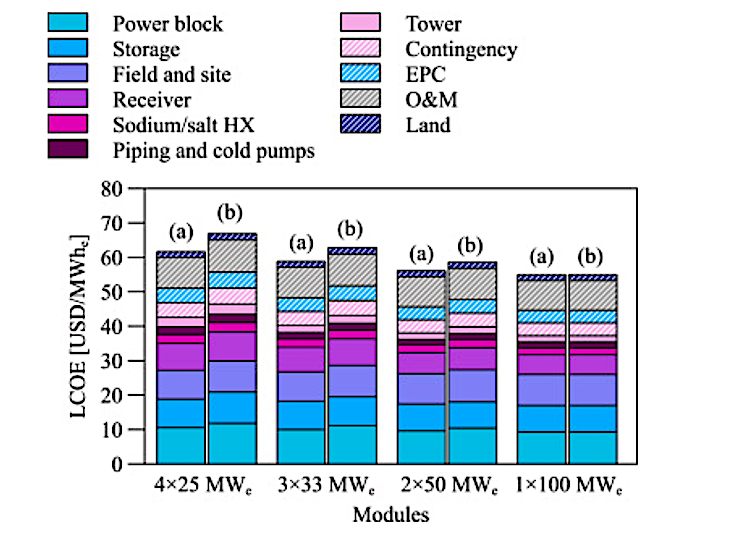
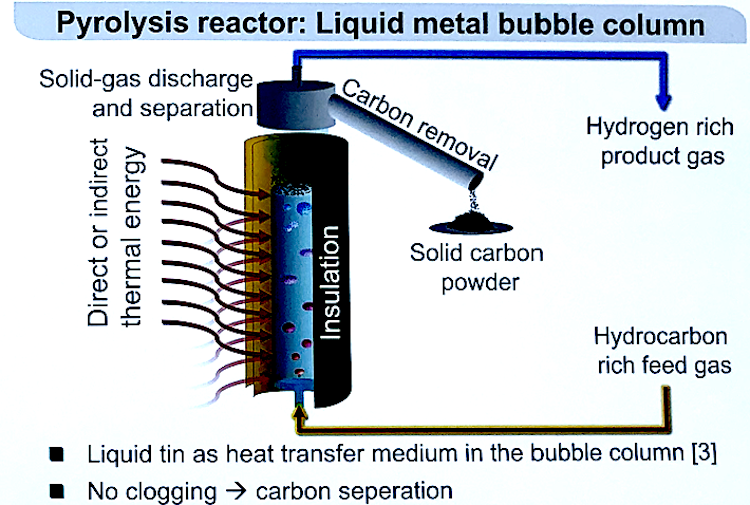
Could the Chemical Potential Analysis method work better than the van’t Hoff method?
NREL’s Stephan Lany proposes a new common language to help solar thermochemistry researchers select the redox materials that will most efficiently generate green hydrogen. He has published the proposal in the Journal of the American Chemical Society Chemical Potential Analysis as an Alternative to the van’t Hoff Method: Hypothetical Limits of Solar Thermochemical Hydrogen.
Why this matters
Solar thermochemical splitting of water and carbon dioxide is a promising method for converting solar energy into fuels on a large scale, potentially adding another renewable technology to substitute for fossil-fuel-generated hydrogen and aviation fuel.
Ceria (CeO2) is currently the benchmark oxide material for water splitting with a two-step reduction and oxidation cycle. (Here, reduction is the release of oxygen from the solid oxide, and oxidation is the replenishment of O from H2O steam, generating H2). However, the hydrogen production of CeO2 is very limited unless the reduction is performed at very high temperatures of 1500°C or even higher.
Although some solar researchers approach the problem by designing reactors and receivers capable of these higher temperatures, finding a new redox material that might be as effective as ceria in splitting water during oxidation but releasing more oxygen during reduction below 1400°C could be a game-changer for these solar fuels.
“There are tens of thousands of oxides known in their composition and crystal structure,” he explained in a call from Colorado. “But not all of them have been characterized and evaluated whether they would work.However, how do we know how well a newly predicted candidate material would perform?”
Lany proposes a different analysis method to make sure theory and experiment are on the same page and are able to create a positive feedback loop, which is a prerequisite for developing new materials or finding ways to improve ceria.
How the traditional method of analysis works
The traditional method of analysis for determining reaction enthalpies and entropies for processes like solar thermochemical fuel production is the van’t Hoff method, which Lany describes as somewhat outdated and obsolete.
This method’s limitation is that it mixes the properties of the probe gas with those of the oxides being studied, which can obscure the view on the properties that matter.
“In thermochemistry, what we look into, from a formal kind of chemistry standpoint, is the free energy,” Lany explained.
“The free energy is the kind of governing equation and it’s a very general rule in chemistry that any process works to minimize the free energy. This is the driving force for all reactions. And if we look into what is the free energy of the reduction reaction, then there is a part that relates to the solid state properties. That means the solid oxide, in its oxidized and its reduced form. There’s also a part coming from the fact that we bring oxygen out of the oxide into the gas phase. The free energy has two contributions, the enthalpy and the entropy. And the total free energy is enthalpy minus temperature times entropy. Van’t Hoff separates the free energy into the enthalpy and the entropy, but it does not separate the part that comes from the oxide in its solid state and the part that comes from the oxygen in the gas phase.”
Why the chemical potential analysis method is better
Instead, Lany takes a new approach, the chemical potential analysis method, which helps better understand the defect mechanisms and their impact on material behavior. The chemical potential method does not only separate solid and gas phase contributions, but also allows the analysis of the temperature dependence of enthalpy and entropy, which can reveal important details needed to design better materials.
“This is a more modern way to process the data using more freely definable functional relationships. The idealized linear relationship in the traditional van’t Hoff method was very useful back in the days when data analysis was performed with graph paper and ruler, but it holds only for constant enthalpy and entropy. With the chemical potential method, we can relatively easily determine the temperature dependencies,” he said.
This new approach allows a more detailed analysis of the materials’ behavior, providing important clues about the chemical and physical mechanisms that determine the thermochemical properties.
Lany’s study tested this new analysis method on three computational model systems including ceria, which shows unique behavior due to the way the electrons can redistribute when O atoms are removed from the crystal lattice. This process is temperature dependent and is ultimately responsible for the ability of CeO2 to produce concentrated H2/H2O mixtures whereas most other oxides split water, if at all, then only under very dilute conditions like 1/1000.
Tracking the temperature dependence of enthalpy and entropy is a way to recognize the behavior that makes CeO2 so special. Lany’s hope is that this could become the new common language to make the connection between experimental observations and theoretical models. He says: “I think this is a very useful idea for people in thermochemistry who do thermogravimetric analysis and try to distill insights about the reduction mechanism in the materials from their measurements.”
A balancing act
Although modifying ceria or discovering new oxides could improve performance, gains in the reduction step often worsen the oxidation step in redox cycles, limiting hydrogen production. The goal is to balance reduction and oxidation properties to optimize material performance for solar fuel generation.
“I take ceria as a baseline material, and then I say, Okay, what kind of properties should a different material have to improve over the behavior of ceria,” he said.
“I was just considering if we could design materials that are similar but different from ceria – what could we achieve for water splitting with those materials? I tend to be on the side of new materials, but I would not exclude the possibility of modification of ceria either; which is usually something like alloying or doping the material, where we add one or more additional elements.”
Lany’s paper describes how the proposed new common language is used to connect the different defect and materials properties to thermochemical properties (temperature dependence of enthalpy and entropy) and from there to water splitting performance of existing and hypothetical materials, specifically looking at both total H2 output and the H2/H2O ratio in the oxidation step.
More reading: Chemical Potential Analysis as an Alternative to the van’t Hoff Method: Hypothetical Limits of Solar Thermochemical Hydrogen
3D-printed ceria is a game-changer to increase solar fuel efficiency
How can Synhelion’s solar receiver achieve such high temperatures?